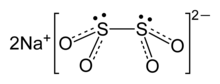
Sodium dithionite
跳至導覽
跳至搜尋
| |

| |

| |
| 號名 | |
|---|---|
| 其他號名
D-Ox, Hydrolin, Reductone
sodium hydrosulfite, sodium sulfoxylate, Sulfoxylate Vatrolite, Virtex L Hydrosulfit, Prayon Blankit, Albite A, Konite Zepar, Burmol, Arostit | |
| 識別號 | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number | 231-890-0 |
PubChem CID
|
|
| RTECS number | JP2100000 |
| UNII | |
| UN number | 1384 |
| 性質 | |
| 焦2S2O4 | |
| Mole質量 | 174.107 g/mol (anhydrous) 210.146 g/mol (dihydrate) |
| 外貌 | white to grayish crystalline powder light-lemon colored flakes |
| 氣味 | faint sulfur odor |
| 密度 | 2.38 g/cm3 (anhydrous) 1.58 g/cm3 (dihydrate) |
| 熔點 | 52 °C (126 °F; 325 K) |
| 沸點 | Decomposes |
| 18.2 g/100 mL (anhydrous, 20 °C) 21.9 g/100 mL (Dihydrate, 20 °C) | |
| 溶解度 | slightly soluble inalcohol |
| 危險 | |
| GHS pictograms |  
|
| GHS signal word | Danger |
| NFPA 704 | |
| 引火點 | 100 °C (212 °F; 373 K) |
| 200 °C (392 °F; 473 K) | |
| 關連化合物 | |
其他 陰離子
|
Sodium sulfite Sodium sulfate |
關連化合物
|
Sodium thiosulfate Sodium bisulfite Sodium metabisulfite Sodium bisulfate |
除了特別指出,資料是根據物質的標準狀態 (佇25 °C [77 °F ], 100 kPa). | |
| Infobox 參照 | |
Sodium dithionite (mā kiò-tsòsodium hydrosulfite;
註解 [修改]
- ↑ Weinrach, J. B.; Meyer, D. R.; Guy, J. T.; Michalski, P. E.; Carter, K. L.; Grubisha, D. S.; Bennett, D. W. (1992). "A structural study of sodium dithionite and its ephemeral dihydrate: A new conformation for the dithionite ion". Journal of Crystallographic and Spectroscopic Research. 22 (3): 291–301. doi:10.1007/BF01199531. (英語)
參考 文 hèn[修改]
- "Sulfites, Thiosulfates, and Dithionites". Ullmann's Encyclopedia of Industrial Chemistry (6th pán.). Weinheim: Wiley-VCH. 2003. pp. 7–11. doi:10.1002/14356007.a25_477. ISBN 978-3-527-30385-4. (英語)
- Лидин Р.А。 и др。 Химические свойства неорганических веществ: Учеб。 пособие для вузов。 — 3-е изд., испр。 — М.: Химия, 2000。 — 480 с。 — ISBN 5-7245-1163-0。(俄語)
參娃 ̽t[修改]
- Pang-bô͘:Ill (
機格 (技師 ̽t標準 )/Specification (technical standard)/諸元 /諸元) - Pang-bô͘:Ill (
深桃 理皇 /The Scarlet Empress) - Pang-bô͘:Ill (Smolnii
哈 ̽k-īñ/Смо́льный институ́т) - Pang-bô͘:Ill (Hèn
發 重大 信朝 19條 (1911)/Nineteen Articles) - Pang-bô͘:Ill (Pen
程 工具 /Programming tool)
